Abstract
Here we report the synthesis of a crystalline micrometric-sized hexagonal-shaped Ni2+-Fe3+ LDH by following a modified homogeneous precipitation method. The exfoliation of the material in formamide leads to stable suspensions of hexagonal nanometric sheets, which have been extensively characterized. Our data confirm that the intrinsic properties of the bulk material are retained by these segregated nanosheets, thus opening the door for their use in the development of layered multifunctional materials.
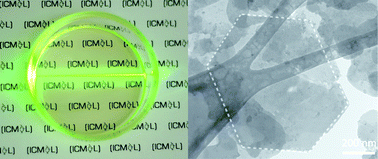
Exfoliation of layered hydroxides, defined as the segregation of these lamellar inorganic solids into single entities through soft chemistry methods,1 can be regarded as a versatile synthetic route to prepare stable suspensions of sheets, typically exhibiting homogeneous micrometric lateral size and nanometric thickness. Concerning materials science, these 2D nanosheets offer very interesting features: (a) owing to the nanometric thickness of these layers, they may exhibit new physical properties different from the bulk; (b) compared to other low dimensional nanomaterials (0D or 1D), their two-dimensional structure facilitates their processability. In fact, these layers can be re-assembled into highly oriented films by employing Langmuir–Blodgett (LB) or Layer-by-Layer (LbL) techniques;2 (c) they can lead to the design of new multifunctional materials combining sophisticated physical properties through the rational choice of the constituting building blocks and the precise control of their arrangement in the solid state.3
Among these layered materials, we were particularly interested in the layered double hydroxides (LDHs) compounds. The structure of these anionic clays is closely related to that of the brucite, Mg(OH)2, with partial substitution of some of the divalent positions with M3+ cations. This fact generates an excess of positive charge in the layers which must be balanced by the presence of anions and water molecules interleaved between the layers according to the formula: [MII1−xMIIIx(OH)2](An−)x/n·yH2O. In this way, their exfoliation in organic solvents produces steady colloidal suspensions of positively charged nanosheets,4 which in the presence of anionic components, can be further re-assembled into sandwich-like layered materials as result of the presence of attractive Coulombic interactions.5 In addition, LDHs offer a wide plethora of intrinsic magnetic, optical or catalytic properties,6 which ultimately will be retained by the segregated layers and transferred to these layered super-structures. Concerning magnetism, we recently reported the presence of spontaneous magnetization below 15 K in Ni2+-Fe3+ LDHs prepared by the traditional coprecipitation method, as result of the combination of strong antiferromagnetic superexchange interactions between the in-plane ions mediated by the hydroxyl bridge and much weaker dipolar interactions between the layers.7 Unfortunately, the synthesis of crystalline Fe3+-based LDHs with hexagonal morphology in an extendable controlled manner has been elusive so far. More generally, the synthesis of non-Al3+-based LDHs is very disfavoured mainly due to the specific amphoteric behaviour of Al(OH)3. Except for remarkable efforts,8 the use of traditional coprecipitation methods, based on the direct combination of the constituting ions in a basic medium, has just led to the isolation of amorphous phases. Although these materials can indirectly enhance its crystallinity after an additional hydrothermal treatment, they cannot be effectively exfoliated.9
Here we report how, by following a modified homogeneous precipitation method, whose novelty resides in the use of the chelating agent triethanolamine (TEA; C6H15NO3) as auxiliary reagent, permits the synthesis of a highly crystalline micrometric-sized hexagonal-shaped [Ni1-xFex(OH)2](CO3)x/2 (x = 0.25) LDH. Besides, this material can be readily ion-exchanged with nitrate anions through the so-called “acid-salt treatment”,10 permitting its quantitative exfoliation in formamide. Regarding the potential magnetic functionality of these Ni2+Fe3+-LDH nanosheets,7 this work paves the way for their use in the design of advanced multifunctional materials.